This title might make you gasp and go “My goodness they are doing what now? Injecting humans with viruses?”
Relax.
These viruses aren’t your standard HIV or flu or Ebola viruses you hear about in the news that cause sickness in humans. These viruses are bacterial viruses – also known as bacteriophages (literally “bacteria eaters”) or simply phages for short.
How does it work?
Phages are no different than other types of viruses: they require a host to replicate. In other words, they enter a bacterial cell, make copies of themselves inside it (using the bacterium’s resources) and eventually burst out of the cell; killing it in the process. These new phages then latch onto nearby bacteria (from the same species) and begin the process once again.
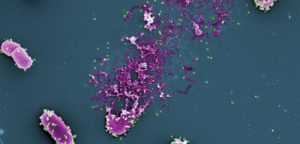
Bacteriophage (in green) causing a bacterial cell (in purple) to burst, releasing phage particles.
Imagine you have a hoard of disease-causing or antibiotic-resistant bacteria like Acinetobacter baumanniiinside your body. By delivering phages thatspecifically infect Acinetobacter baumanniiat the site of infection, they can kill it while leaving other “good bacteria” (also known as microbiome) intact.
Phage therapy is not a new field of research. In fact, it is an outdated field. Phages were first discovered in the early 1900s. They enjoyed a brief moment of spotlight, before Sir Alexander Fleming discovered penicillin. Phages have been used to treat bacterial infections since 1942. Since then, the discovery of various antibiotics compounds has rendered phage therapy obsolete. However in the face of increasing antimicrobial resistance and the emergence of “superbugs” – bacterial strains that are resistant to many types of antibiotics – researchers are once more looking at phage therapy as an alternative to combat bacterial infection.
That sounds cool! So why have we not started using them already?
There are many factors to consider before commencing treatment and it is no exception with phage therapy. Firstly, since phages only replicate inside a bacterium, their preparation would inevitably include the bacteria itself. That means that in the downstream processing, there needs to be a step to purify these phage particles from other bacterial proteins that are released in the process. Failure to do so may cause serious immunological reactions.
Secondly, as phages are usually species- or even strain-specific, the bacterial species causing the infection needs to be determined before any therapy can be administered.
Oh, okay. But won’t the bacteria become resistant to the phages at some point during the treatment?
They can! However, phages are also “biological beings” – so to speak (to call them “living” would spark another lengthy debate). As opposed to an antibiotic compound, phages can evolve to overcome resistance.
There are also other strategies to overcome this problem. One of them is to identify multiple phage strains that can infect the bacteria so that if it becomes immune to one phage strain, other strains are still capable of infecting it.
Another way is to combine phage therapy with antibiotic treatment. Becoming phage-resistant or antibiotic-resistant takes a huge toll on the bacteria and consumes cellular resources. Simply put, bacteria that resist antibiotics may be more susceptible to phage infection, and vice versa. With this knowledge, scientists can devise a synergistic treatment plan that combines the usage of antibiotics and phage.
But wait, I’ve heard that phages can sometimes transfer antibiotic-resistant genes between bacteria. Isn’t that a problem?
Yes, it is. To get into that, we need to go a bit deeper into phage biology. Phages have 2 types of life cycles: lytic and lysogenic. A lytic life cycle is when the phage alway skills the bacterium that it infects, while a lysogenic life cycle is when the phage integrates its genome into the bacterium’s. The gene transfer only happens with lysogenic phages. Therefore, to circumvent this problem, only lytic phages are used for therapy.
Oh wow, that sounds cool! So where can I get phage therapy?
Are you having a serious bacterial infection that cannot be treated with antibiotics? Just kidding!
Sadly there are not many centers that provide or offer this type of treatment at the moment. Phage therapy is still a blooming – albeit old – field and there are many regulatory hurdles overcome.
Globally, there are a few institutions specialising in research for phage therapy, for example the George Eliava Institute of Bacteriophage, Microbiology and Virology in Georgia; and Ludwik Hirszfeld Institute of Immunology and Experimental Therapy in Poland.
Worldwide acceptance for phage therapy is still lacking and there remains a large gap to fill for pushing forward this kind of therapy. However, there are limited reports of large-scale clinical trials, where most of the documented cases are considered as a last resort.
It is pretty safe to say that phage therapy might, in the near future, become one of the accepted treatments for bacterial infections!
References:
Article sources: https://doi.org/10.1016/j.chom.2019.01.014
https://www.id-hub.com/2017/08/04/phage-therapy-vivo-important-hosts-immune-response/
Video source: https://www.youtube.com/watch?v=YI3tsmFsrOg
Image source: https://www.id-hub.com/2017/08/04/phage-therapy-vivo-important-hosts-immune-response/
This article was written by Ahmad Tarmizi Abdul Halim who has a BSc. In Biotechnology and Molecular Bioscience, Rochester Institute of Technology (RIT, 2015) and is currently doing MSc. At Universiti Tunku Abdul Rahman (UTAR), Kampar. His field of study is microbiology and protein purification.
[This article belongs to The Malaysian Medical Gazette. Any republication (online or offline) without written permission from The Malaysian Medical Gazette is prohibited.]
